Equilibrium Constant and Reaction Quotient
Help Questions
GRE Subject Test: Chemistry › Equilibrium Constant and Reaction Quotient
Which of the following is true regarding the solubility product constant, 
The concentration of substance 
As more of substance 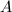


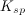
Explanation
To determine the solubility product constant, we only need the concentrations and coefficients of the ions. The effective concentration of any pure substance (solid, liquid, or gas) is equal to one by definition, so does not influence the value of 
The units of the solubility product constant will depend on the coefficients of the products. 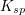

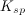
![K_{sp}=[C]^c[D]^d](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/73718/gif.latex)
![K_{sp}=[C]^c[D]^d](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/100575/gif.latex)