Alkane Chemistry
Help Questions
GRE Subject Test: Chemistry › Alkane Chemistry

How could you brominate the compound?
Bromine and UV light
Hydrobromic acid
Bromine and peroxides
Bromine gas
None of these
Explanation
The given molecule is an alkane. The only way to brominate an alkane is with bromine gas and UV light. The energy from the light serves to creat two radical bromines. These radicals are capable of bonding with alkanes. If the given compound were an alkene, either hydrobromic acid or bromine and peroxides would work.
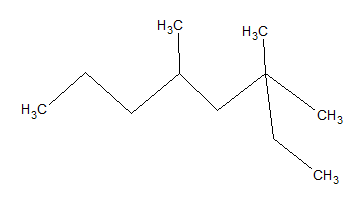
What is the IUPAC name of the given molecule?
3,3,5-trimethyloctane
2,2,4-trimethyloctane
3,3,5-trimethylnonane
4,6-dimethyl-6-ethylpentane
None of these
Explanation
The longest carbon chain that can be formed is eight carbons. The base molecule is octane.
Using IUPAC rules, substituents should have the lowest possible numbers; thus, we start counting carbons from the right side rather than the left. If you count from the correct side, there are two methyl groups on carbon 3 and one on carbon 5. Thus, the name of the moleculue is 3,3,5-trimethyloctane.
Which of the following can reduce an alkene to an alkane?
H2/Pd and H2/Raney nickel
Lithium aluminum hydride (LiAlH4) and H2/Pd
H2/Raney nickel
H2/Pd
Lithium aluminum hydride (LiAlH4)
Explanation
Neither lithium aluminum hydride, nor sodium borohydride will reduce C–C double bonds.
H2/Raney nickel and H2/Pd can each (individually) reduce an alkene to an alkane. Since both H2/Raney nickel and H2/Pd can reduce the alkene, the answer is both of those reagents. This is a catalytic hydrogenation reaction, and H2/Raney nickel not only reduces C–C double bonds, but also carbonyl compounds.
Identify the major organic product expected from the acid-catalyzed dehydration of 2-methyl-2-pentanol.
2-methyl-2-pentene
None of the other answers
2-methyl-1-pentene
3-methyl-1-pentene
cis-3-methyl-2-pentene
Explanation
The initial compound is a five-carbon alkane chain with methyl and hydroxy groups on the second carbon. Dehydration involves the hydrogenation of the hydroxy group. That group then leaves, and a double bond is formed. Zaitsev's rule states that double bonds are more stable on more highly substituted carbons. The double bond forms across carbons two and three.