Molecules
Help Questions
AP Chemistry › Molecules
How many lone pairs of electrons exist on the following molecule?
BF3
1
0
9
10
11
Explanation
.. ..
:F — B — F: Boron can have a violated octet (6 e-) and each F has 3 lone pairs
.. | .. for a total of 9 pairs of unpaired electrons
:F:
..
How many lone pairs of electrons exist on the following molecule?
BF3
1
0
9
10
11
Explanation
.. ..
:F — B — F: Boron can have a violated octet (6 e-) and each F has 3 lone pairs
.. | .. for a total of 9 pairs of unpaired electrons
:F:
..
Based on VSEPR theory and electron configurations, which of the following molecules is not matched with its correct geometry?



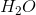

Explanation

What is the molecular geometry of 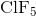
Square pyramidal
Square planar
Octahedral
Trigonal pyramidal
Tetrahedral
Explanation
There are six electron domains in 


What is the Lewis dot structure for 





Explanation
When drawing a Lewis dot structure, we are always trying to reach an electron count where all atoms involved are stable and (usually) have full octets. We are also trying to estabilsh a structure in which we have the smallest formal charge possible. The general rule is first to draw out all of the elements involved and their valence electrons, then start piecing them together trying to reduce the formal charge and get all elements involved to an octet. There are a couple exceptions to the octet rule.
In this case, boron actually has an incomplete octet. Though there are resonance forms in which boron has a full octet, when you calculate the formal charge of these configurations it will not be zero.
What is the Lewis dot structure for 





Explanation
When drawing a Lewis dot structure, we are always trying to reach an electron count where all atoms involved are stable and (usually) have full octets. We are also trying to estabilsh a structure in which we have the smallest formal charge possible. The general rule is first to draw out all of the elements involved and their valence electrons, then start piecing them together trying to reduce the formal charge and get all elements involved to an octet. There are a couple exceptions to the octet rule.
In this case, boron actually has an incomplete octet. Though there are resonance forms in which boron has a full octet, when you calculate the formal charge of these configurations it will not be zero.
A compound is comprised of sodium and element X. The compound has a molar mass of 

Molecular:
Empirical:
Molecular:
Empirical:
Molecular:
Empirical:
Molecular:
Empirical:
None of these are correct
Explanation
Use the molar mass and percent by mass of sodium to find the moles of sodium in the compound.
Since the compound consists of only sodium and element X, we can subtract to find the percent by mass of element X.
Use the percent by mass to find the mass per mole of compound of element X.
Because there is a 1:1 ratio, between sodium and element X, they must have an equal number of moles in the compound. Since there are 2 moles of sodium, there must also be 2 moles of element X. Divide the mass of element X by the moles of element X to find its atomic mass and its identity.
By consulting a periodic table, we can find that element X is oxygen.
Therefore element X is Oxygen.
Therefore, the molecular formula is 

Which of the following compounds contains an atom that does NOT satisfy the octet rule?
I.
II.
III.
IV.
I and IV
I, II, and IV
II and III
I and II
IV only
Explanation
BCl3 only has six electrons around boron, while NO2 (with an odd number of electrons) would have only 7 electrons around the central nitrogen.
Which of the following compounds contains an atom that does NOT satisfy the octet rule?
I.
II.
III.
IV.
I and IV
I, II, and IV
II and III
I and II
IV only
Explanation
BCl3 only has six electrons around boron, while NO2 (with an odd number of electrons) would have only 7 electrons around the central nitrogen.
A compound is comprised of sodium and element X. The compound has a molar mass of 

Molecular:
Empirical:
Molecular:
Empirical:
Molecular:
Empirical:
Molecular:
Empirical:
None of these are correct
Explanation
Use the molar mass and percent by mass of sodium to find the moles of sodium in the compound.
Since the compound consists of only sodium and element X, we can subtract to find the percent by mass of element X.
Use the percent by mass to find the mass per mole of compound of element X.
Because there is a 1:1 ratio, between sodium and element X, they must have an equal number of moles in the compound. Since there are 2 moles of sodium, there must also be 2 moles of element X. Divide the mass of element X by the moles of element X to find its atomic mass and its identity.
By consulting a periodic table, we can find that element X is oxygen.
Therefore element X is Oxygen.
Therefore, the molecular formula is 

















