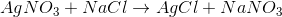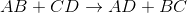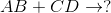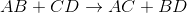Types of Reactions
Help Questions
AP Chemistry › Types of Reactions
Which of the following describes an oxidation-reduction reaction?
The transfer of electrons between species, and the subsequent changes in oxidation states
A reaction in which one species molecule is oxidized then reduced
A type of chemical reaction that always involves acids and bases
None of these
A type of reaction in which one reactant is broken down into two products
Explanation
In an oxidation reaction, one species is oxidized (loses electrons). During a reduction reaction, one species is reduced (gains electrons). These transfers of electrons results in the change of oxidation states. One way to help you remember which reaction is which, is by using the mnemonic: OIL RIG - Oxidation Is Loss of electrons; Reduction Is Gain of electrons.
Which of the following describes an oxidation-reduction reaction?
The transfer of electrons between species, and the subsequent changes in oxidation states
A reaction in which one species molecule is oxidized then reduced
A type of chemical reaction that always involves acids and bases
None of these
A type of reaction in which one reactant is broken down into two products
Explanation
In an oxidation reaction, one species is oxidized (loses electrons). During a reduction reaction, one species is reduced (gains electrons). These transfers of electrons results in the change of oxidation states. One way to help you remember which reaction is which, is by using the mnemonic: OIL RIG - Oxidation Is Loss of electrons; Reduction Is Gain of electrons.
What is the type of reaction seen below?
Double-replacement
Single-replacement
Addition
Decomposition
Explanation
By looking at the reaction, we see that the cations for the reactants have their anions switched in the products. This means that the reaction follows the general outline:
This is an example of a double-replacement reaction.
Addition reactions convert two reactants to a single product, while decomposition reactions convert a single reactant to multiple products. Single-replacement reactions only switch one cation-anion pair.
Determine which compound or ion is the oxidizing agent in the given reaction.
Explanation
The oxidizing agent is the element that causes the other element to be oxidized, and is itself being reduced in the equation. Reduction is defined as the gain of electrons, meaning the element would get "more negative."
In this reaction, aluminum transitions from an oxidation state of 3+ to an oxidation state of 0. This indicates a reduction, as the atom has gained more electrons and reduced charge. 
What is the type of reaction seen below?
Double-replacement
Single-replacement
Addition
Decomposition
Explanation
By looking at the reaction, we see that the cations for the reactants have their anions switched in the products. This means that the reaction follows the general outline:
This is an example of a double-replacement reaction.
Addition reactions convert two reactants to a single product, while decomposition reactions convert a single reactant to multiple products. Single-replacement reactions only switch one cation-anion pair.
Determine which compound or ion is the oxidizing agent in the given reaction.
Explanation
The oxidizing agent is the element that causes the other element to be oxidized, and is itself being reduced in the equation. Reduction is defined as the gain of electrons, meaning the element would get "more negative."
In this reaction, aluminum transitions from an oxidation state of 3+ to an oxidation state of 0. This indicates a reduction, as the atom has gained more electrons and reduced charge. 
Predict the products for the following reaction if it were double displacement.
Explanation
In a double displacement reaction, or double replacement reaction, opposite ions combine. What this means is that the cation of one compound will recombine and bond to the anion of the other compound and vice versa.
In this example, we have to remember the ideal convention of cations being written before the anion in the compound. So in AB, we can presume A being the cation and B being the anion. The same goes for CD - C is the cation and D is the anion.
With this in mind, we can now easily see how the replacement would be possible.
If A is a cation, and was originally bound to B (anion), the only other anion it is left with to bind is D. If C is a cation, and was originally bound to D (anion), it is only left with the option of binding to the now-free B anion.
That's why it is:
Predict the products for the following reaction if it were double displacement.
Explanation
In a double displacement reaction, or double replacement reaction, opposite ions combine. What this means is that the cation of one compound will recombine and bond to the anion of the other compound and vice versa.
In this example, we have to remember the ideal convention of cations being written before the anion in the compound. So in AB, we can presume A being the cation and B being the anion. The same goes for CD - C is the cation and D is the anion.
With this in mind, we can now easily see how the replacement would be possible.
If A is a cation, and was originally bound to B (anion), the only other anion it is left with to bind is D. If C is a cation, and was originally bound to D (anion), it is only left with the option of binding to the now-free B anion.
That's why it is:
What process could be used to describe the conversion of 

Reduction
Oxidation
Combustion
Neutralization
Radioactive decay
Explanation
The process illustrated is the reduction of the chromium atom. Reduction occurs when electrons are gained, causing the oxidation number (charge) of the atom to decrease. We can examine this process by looking at the oxidations numbers of each atom involved.
Oxygen always has an oxidation number of 

The initial oxidation state of chromium is 
Following the reaction, chromium has a charge of 

During the process, the chromium atom went from an oxidation state of 

What process could be used to describe the conversion of 

Reduction
Oxidation
Combustion
Neutralization
Radioactive decay
Explanation
The process illustrated is the reduction of the chromium atom. Reduction occurs when electrons are gained, causing the oxidation number (charge) of the atom to decrease. We can examine this process by looking at the oxidations numbers of each atom involved.
Oxygen always has an oxidation number of 

The initial oxidation state of chromium is 
Following the reaction, chromium has a charge of 

During the process, the chromium atom went from an oxidation state of