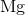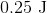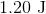Physical Chemistry
Help Questions
Physical Chemistry › Physical Chemistry
In an atom or molecule, why can't two electrons have the same four electronic quantum numbers?
The Pauli Exclusion Principle
Heisenberg Uncertainty Principle
Harmonic Reaction Orders
Kinetic energy operator
The first law of thermodynamics
Explanation
The Pauli Exclusion Principle explains various phenomena such as the structure of atoms and how different atoms combine to share electrons. When you have two electrons that are located in the same orbital, the quantum numbers n, l and ml are the same. However, ms will be different. Two electrons cannot have the same four electronic quantum numbers because no more than two electrons may occupy an orbital, and if they do, the spin of one must cancel the spin of the other so their spins will have a zero net spin angular momentum.
What is the complete ground state electron configuration for the magnesium atom?
1s22s22p63s2
1s22s23s2
1s42p63s2
1s22s22p23s2
1s22s22p63s6
Explanation
Magnesium has an atomic number of 12, so the total number of electrons in its configuration should add up to twelve. The maximum number of electrons in the s subshell is two. Of all the answer choices, only 1s22s22p63s 2 fits the criteria. The sum of the exponent values is 12, matching the atomic number of magnesium, and the number of electrons in the s and p subshells matches the maximum amount possible.
Which of the following trends decreases as you move from left to right on the periodic table?
Atomic radius
Electronegativity
Electron affinity
Ionization energy
Explanation
Although it may seem counterintuitive, atomic radius does decrease from left to right on the periodic table. The reason for this is because the added positive charge in the nucleus causes the elctrons to be pulled more strongly towards the center, which decreases the atomic radius.
Which of the following is not an intensive property?
Volume
Temperature
Density
Melting point
Pressure
Explanation
Intensive properties are not dependent on the amount of substance. Melting point, pressure, temperature, and density are some examples of intensive properties. Therefore, volume of a substance is not an example of an intensive property, rather, it is an extensive property which depends on the amount of substance. Some other examples of extensive properties include weight, energy, and electric charge.
Which of the following represents the alkaline earth metal with the greatest atomic number?
Explanation
Alkaline earth metals are group II elements; among them, radium has the largest atomic number.
Which of the following represents the alkaline earth metal with the greatest atomic number?
Explanation
Alkaline earth metals are group II elements; among them, radium has the largest atomic number.
Which of the following are true regarding 

I. Both 

II. In a given shell, 
III. 

I and III
I only
III only
I and II
Explanation
Orbitals are regions in an electron shell where electrons might be located. There are several types of orbitals such as 










Which of the following is not an intensive property?
Volume
Temperature
Density
Melting point
Pressure
Explanation
Intensive properties are not dependent on the amount of substance. Melting point, pressure, temperature, and density are some examples of intensive properties. Therefore, volume of a substance is not an example of an intensive property, rather, it is an extensive property which depends on the amount of substance. Some other examples of extensive properties include weight, energy, and electric charge.
In an reaction, the products are more stable than the reactants; in an reaction the reactants are more stable than the products.
exergonic . . . endergonic
endergonic . . . exergonic
endergonic . . . endergonic
exergonic . . . exergonic
Explanation
Exergonic reactions release energy; therefore, the energy of products is lower than that of the reactants. Endergonic reactions consume energy; therefore, the energy of products is greater than that of the reactants. In other words, exergonic reactions are spontaneous, while endergonic reactions are nonspontaneous, and require the net input of energy to drive the reaction.
A sample of an ideal gas is initially at a volume of 



Explanation
The expression for the relationship between heat (


The work (


Plugging work (
Plugging the values given into the internal energy equation gives: