Reactions by Reactant
Help Questions
Organic Chemistry › Reactions by Reactant

What is the product of the given reaction?




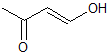
Explanation
This is a basic Claisen condensation reaction in which the 
 .
.
The attack at the ester carbonyl leads to the leaving of 

What is the product of the given reaction?





Explanation
This is a basic Claisen condensation reaction in which the 
 .
.
The attack at the ester carbonyl leads to the leaving of 

What is the product of the given reaction?





Explanation
This is a basic Claisen condensation reaction in which the 
 .
.
The attack at the ester carbonyl leads to the leaving of 
Predict the major product of the following reaction.

I
II
III
IV
Explanation
Alkenes are saturated by hydrogen gas in the presence of a palladium catalyst. This type of reaction is termed catalytic hydrogenation and results in syn addition of hydrogen across a double bond. Molecules II, III, and IV have all undergone oxidation-reduction reactions and do not result from the given conditions. Molecule I is altered only by hydrogenation of the 2-3 double bond and is the correct answer.
What is the product of the following reaction?


II and III
II only
I and II
IV only
Explanation
Treatment of ethyl acetate with methoxide will yield the enolate ion (II). However, side trans-esterification reactions will also result in formation of product III.
What is the product of the following reaction?


II and III
II only
I and II
IV only
Explanation
Treatment of ethyl acetate with methoxide will yield the enolate ion (II). However, side trans-esterification reactions will also result in formation of product III.
Predict the major product of the following reaction.

I
II
III
IV
Explanation
Alkenes are saturated by hydrogen gas in the presence of a palladium catalyst. This type of reaction is termed catalytic hydrogenation and results in syn addition of hydrogen across a double bond. Molecules II, III, and IV have all undergone oxidation-reduction reactions and do not result from the given conditions. Molecule I is altered only by hydrogenation of the 2-3 double bond and is the correct answer.
What is the product of the following reaction?


II and III
II only
I and II
IV only
Explanation
Treatment of ethyl acetate with methoxide will yield the enolate ion (II). However, side trans-esterification reactions will also result in formation of product III.
Predict the major product of the following reaction.

I
II
III
IV
Explanation
Alkenes are saturated by hydrogen gas in the presence of a palladium catalyst. This type of reaction is termed catalytic hydrogenation and results in syn addition of hydrogen across a double bond. Molecules II, III, and IV have all undergone oxidation-reduction reactions and do not result from the given conditions. Molecule I is altered only by hydrogenation of the 2-3 double bond and is the correct answer.

What is the product of the reaction given?




Explanation
Below is the mechanism for the reaction given which is called the McMurry Reaction. It is a titanium (Ti) prompted pinacol coupling followed by deoxygenation.
