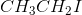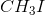Organic Chemistry
Help Questions
Organic Chemistry › Organic Chemistry
What is the IUPAC name of the following compound?

3-bromo-2-methylcyclohexene
None of the other answers
1-bromo-2-methyl-2-cycloxene
6-bromo-1-methylcyclohexene
2-bromo-1-methylcyclohexene
Explanation
The numbering of the molecule begins across the double bond and goes in the direction where the lowest numbers for substituents can be achieved. Thus, the methyl group will be on carbon two and the bromine on carbon 3. Substituents are ordered alphabetically. The base molecule is a six-membered ring with one double bond. This is called a cyclohexene.
Which of the following substrates would have the fastest reaction rate for an SN1 mechanism?
Explanation
The SN1 mechanism involves the formation of a carbocation intermediate in the rate-determining step. The most stable carbocation will produce the fastest reaction. We can immediately eliminate any answer choices that will produce primary or secondary carbocations, since a tertiary carbocation will be much more stable. When comparing tertiary carbocations, larger and more electronegative substituents will allow for more charge stabilization.
Since the tertiary carbocation formed by the dissociation of iodide from 
What is the IUPAC name of the following compound?

3-bromo-2-methylcyclohexene
None of the other answers
1-bromo-2-methyl-2-cycloxene
6-bromo-1-methylcyclohexene
2-bromo-1-methylcyclohexene
Explanation
The numbering of the molecule begins across the double bond and goes in the direction where the lowest numbers for substituents can be achieved. Thus, the methyl group will be on carbon two and the bromine on carbon 3. Substituents are ordered alphabetically. The base molecule is a six-membered ring with one double bond. This is called a cyclohexene.
Which element is present in all organic compounds?
Carbon
Nitrogen
Oxygen
Phosphorous
Explanation
By definition, an organic compound is a compound that contains carbon. Organic chemistry is the study of carbon-containing molecules.

What is the product of the reaction given?




Explanation
In the reaction below, a ketone is protonated by a strong acid. This triggers the deprotonation of the weakly acidic hydrogen bonded to the alpha carbon by a bromide ion and formation of an alkene functional group.



II only
I only
I and II
III only
Explanation
Cyanide is a weak base and a good nucleophile, and the solvent is aprotic; therefore, the 
A compound 

The original compound has no triple bonds.
3 double bonds and 2 rings
3 double bonds and 1 ring
4 double bonds and 1 ring
4 double bonds and 2 rings
None of the other answers
Explanation
Hydrogenation of a double bond involves the bond breaking and a hydrogen being added to each carbon of that double bond. You can tell the number of double bonds by taking the number of hydrogens added and dividing it by 2.
6 added hydrogen divided by 2 is 3 double bonds.
A hydrocarbon with zero degrees of unsaturation and 


What is the IUPAC name for the following compound?

Explanation
The longest carbon chain is 








Assuming that this molecule is planar, determine whether this molecule is aromatic, and name the number of 

Not aromatic, 8 
Aromatic, 8 
Aromatic, 6 
Non aromatic, 6 
Explanation
There is no p orbital surrounding the Boron atom, so the ring does not have a fully conjugated pi system. In addition, there are 8 
Ephedrine (shown below) contains what type of amine?

Secondary
Primary
Tertiary
Quaternary
Neutral
Explanation
A secondary amine is an amine (nitrogen atom) that is attached to two carbon-containing groups (alkyl groups or aryl groups). The nitrogen in ephedrine is attached to two alkyl groups, making it a secondary amine.
Primary amines are generally written as