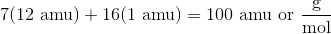Molar Mass
Help Questions
AP Chemistry › Molar Mass
Questions 1 - 1
1
What is the approximate percentage of carbon by mass in heptane, which has the molecular formula 
Explanation
First, calculate the molar mass of heptane, which is
We can calculate the percentage of carbon by mass in heptane by creating a fraction of the mass of carbon over the mass of the entire molecule and converting it into a percentage:
For every 100 g of heptane, 84 g must come from carbon. This means that the percentage of carbon by mass is
AI TutorYour personal study buddy
Question of the DayDaily practice to build your skills
FlashcardsStudy and memorize key concepts
WorksheetsBuild custom practice quizzes
AI SolverStep-by-step problem solutions
Learn by ConceptMaster concepts step by step
Diagnostic TestsAssess your knowledge level
Practice TestsTest your skills with exam questions
GamesLearn through free games