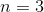Organic Chemistry
Help Questions
GRE › Organic Chemistry
Ephedrine (shown below) contains what type of amine?

Secondary
Primary
Tertiary
Quaternary
Neutral
Explanation
A secondary amine is an amine (nitrogen atom) that is attached to two carbon-containing groups (alkyl groups or aryl groups). The nitrogen in ephedrine is attached to two alkyl groups, making it a secondary amine.
Primary amines are generally written as 

Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

A scientist is studying the rate of reaction 1. He wants to double the rate of the reaction, but is unsure how to increase concentrations of the reactants. Which of the following is true?
Doubling the concentrations of both the hydroxide and the halide will quadruple the reaction rate
Doubling the concentration of the halide only will quadruple the reaction rate
Doubling the concentration of the hydroxide only will quadruple the reaction rate
Neither doubling the concentration of halide, nor doubling the concentration of hydroxide, will quadruple the reaction rate
Reaction rate in this reaction is not determined by concentration
Explanation
Reaction 1 represents an SN2 reaction. The rate limiting step involves both reactants coming together to form a transition state. The rate of this reaction depends on the concentration of both the organic molecule and the nucleophile.
In contrast, reaction 2 is an E1 reaction, in which the rate limiting step is the removal of the leaving group to form a carbocation. In E1 and SN1 reactions, adjusting the concentration of the halide only is enough to affect the rate.
Ephedrine (shown below) contains what type of amine?

Secondary
Primary
Tertiary
Quaternary
Neutral
Explanation
A secondary amine is an amine (nitrogen atom) that is attached to two carbon-containing groups (alkyl groups or aryl groups). The nitrogen in ephedrine is attached to two alkyl groups, making it a secondary amine.
Primary amines are generally written as 

Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

A scientist is studying the rate of reaction 1. He wants to double the rate of the reaction, but is unsure how to increase concentrations of the reactants. Which of the following is true?
Doubling the concentrations of both the hydroxide and the halide will quadruple the reaction rate
Doubling the concentration of the halide only will quadruple the reaction rate
Doubling the concentration of the hydroxide only will quadruple the reaction rate
Neither doubling the concentration of halide, nor doubling the concentration of hydroxide, will quadruple the reaction rate
Reaction rate in this reaction is not determined by concentration
Explanation
Reaction 1 represents an SN2 reaction. The rate limiting step involves both reactants coming together to form a transition state. The rate of this reaction depends on the concentration of both the organic molecule and the nucleophile.
In contrast, reaction 2 is an E1 reaction, in which the rate limiting step is the removal of the leaving group to form a carbocation. In E1 and SN1 reactions, adjusting the concentration of the halide only is enough to affect the rate.
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

A scientist is studying the rate of reaction 1. He wants to double the rate of the reaction, but is unsure how to increase concentrations of the reactants. Which of the following is true?
Doubling the concentrations of both the hydroxide and the halide will quadruple the reaction rate
Doubling the concentration of the halide only will quadruple the reaction rate
Doubling the concentration of the hydroxide only will quadruple the reaction rate
Neither doubling the concentration of halide, nor doubling the concentration of hydroxide, will quadruple the reaction rate
Reaction rate in this reaction is not determined by concentration
Explanation
Reaction 1 represents an SN2 reaction. The rate limiting step involves both reactants coming together to form a transition state. The rate of this reaction depends on the concentration of both the organic molecule and the nucleophile.
In contrast, reaction 2 is an E1 reaction, in which the rate limiting step is the removal of the leaving group to form a carbocation. In E1 and SN1 reactions, adjusting the concentration of the halide only is enough to affect the rate.
Ephedrine (shown below) contains what type of amine?

Secondary
Primary
Tertiary
Quaternary
Neutral
Explanation
A secondary amine is an amine (nitrogen atom) that is attached to two carbon-containing groups (alkyl groups or aryl groups). The nitrogen in ephedrine is attached to two alkyl groups, making it a secondary amine.
Primary amines are generally written as 

Ephedrine (shown below) contains what type of amine?

Secondary
Primary
Tertiary
Quaternary
Neutral
Explanation
A secondary amine is an amine (nitrogen atom) that is attached to two carbon-containing groups (alkyl groups or aryl groups). The nitrogen in ephedrine is attached to two alkyl groups, making it a secondary amine.
Primary amines are generally written as 

Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

A scientist is studying the rate of reaction 1. He wants to double the rate of the reaction, but is unsure how to increase concentrations of the reactants. Which of the following is true?
Doubling the concentrations of both the hydroxide and the halide will quadruple the reaction rate
Doubling the concentration of the halide only will quadruple the reaction rate
Doubling the concentration of the hydroxide only will quadruple the reaction rate
Neither doubling the concentration of halide, nor doubling the concentration of hydroxide, will quadruple the reaction rate
Reaction rate in this reaction is not determined by concentration
Explanation
Reaction 1 represents an SN2 reaction. The rate limiting step involves both reactants coming together to form a transition state. The rate of this reaction depends on the concentration of both the organic molecule and the nucleophile.
In contrast, reaction 2 is an E1 reaction, in which the rate limiting step is the removal of the leaving group to form a carbocation. In E1 and SN1 reactions, adjusting the concentration of the halide only is enough to affect the rate.
Which of the following factors do NOT favor an SN2 reaction of an alkyl halide?
A tertiary carbocation
A primary halide
A good nucleophile
A polar aprotic solvent
Explanation
The way the question is phrased, three answer choices must favor an SN2 reaction, while the "correct" answer is a factor that does not favor, or disfavors an SN2 reaction.
SN2 reactions are bimolecular, and thus their rate of reaction depends on both the substrate and the nucleophile, forming a high energy transition state in which the nucleophile will displace the substate's leaving group at an angle of 180o. The more sterically hindered the compound is, the higher in energy the transition state will be, and the slower the rate of reaction will be. Consequently, SN2 reactions are favored when the leaving group (a halogen in this case) is on a primary carbon center. Additionally, because the reaction is bimolecular, step two of the reaction will NOT occur without a good nucleophile to displace the leaving group. Finally, all SN2 reactions are favored by polar aprotic solvents.
Because SN2 reactions proceed via a transition state, no carbocation intermediate is formed (that happens in SN1 reactions) and therefore the formation of any carbocation favors an SN1 reaction, not an SN2 reaction.

What is the value of 
Explanation
Huckel's rule states that an aromatic compound must have 
If 4n+2=18, then n=4.