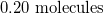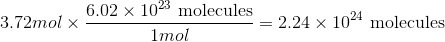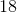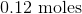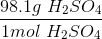Chemistry
Help Questions
GED Science › Chemistry
How many molecules are in 3.72mol of fluorine?
Explanation
Convert from moles to molecules using Avogadro's number:
Use the moles given in the question to solve.
How many molecules are in 3.72mol of fluorine?
Explanation
Convert from moles to molecules using Avogadro's number:
Use the moles given in the question to solve.
How many molecules are in 3.72mol of fluorine?
Explanation
Convert from moles to molecules using Avogadro's number:
Use the moles given in the question to solve.
A student is asked to make 

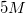
Explanation
Convert liters to mL:
This is the TOTAL volume of the solution. Next, determine how much NaCl to add.
Therefore, 
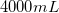
A student is asked to make 

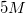
Explanation
Convert liters to mL:
This is the TOTAL volume of the solution. Next, determine how much NaCl to add.
Therefore, 
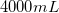
A student is asked to make 

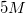
Explanation
Convert liters to mL:
This is the TOTAL volume of the solution. Next, determine how much NaCl to add.
Therefore, 
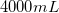
On the periodic table, carbon is atomic number 

Explanation
The standard sub-atomic particles are protons, neutrons, and electrons. Protons and neutrons are found in the nucleus, while electrons move freely around the nucleus. This question is specifically asking about the number of protons and neutrons in an atom of carbon.
Each proton and each neutron has a mass of approximately 

The atomic number refers to the number of protons in the nucleus of the atom. Since the atomic number of carbon is six, there are six protons in the nucleus. For this question, however, we need the total of both the protons and neutrons (not just the protons).
On the periodic table, carbon is atomic number 

Explanation
The standard sub-atomic particles are protons, neutrons, and electrons. Protons and neutrons are found in the nucleus, while electrons move freely around the nucleus. This question is specifically asking about the number of protons and neutrons in an atom of carbon.
Each proton and each neutron has a mass of approximately 

The atomic number refers to the number of protons in the nucleus of the atom. Since the atomic number of carbon is six, there are six protons in the nucleus. For this question, however, we need the total of both the protons and neutrons (not just the protons).
On the periodic table, carbon is atomic number 

Explanation
The standard sub-atomic particles are protons, neutrons, and electrons. Protons and neutrons are found in the nucleus, while electrons move freely around the nucleus. This question is specifically asking about the number of protons and neutrons in an atom of carbon.
Each proton and each neutron has a mass of approximately 

The atomic number refers to the number of protons in the nucleus of the atom. Since the atomic number of carbon is six, there are six protons in the nucleus. For this question, however, we need the total of both the protons and neutrons (not just the protons).
Atomic mass values:
Convert the following mass to moles:
Explanation
To convert grams to moles, use the molecular weight of the molecule. This is found by adding the mass of each atom.
Now we can use the molecular mass to convert the given mass in the question to moles. Make sure to align the values so that the units cancel out.